Electrical conductivity of various substances presentation. Presentation on the topic "electric current in various media"
To create an electric current in a medium it is necessary: - the presence of charged particles in this medium; - external electric field. These conditions are met differently in different environments. Let's consider some of them: - metals; - liquids; - gases. Electric current in liquids
- Solutions of salts, acids and bases that can conduct electric current are called electrolytes.
- The passage of an electric current through an electrolyte is necessarily accompanied by the release of a substance in a solid or gaseous state on the surface of the electrodes.
- The release of a substance on the electrodes shows that in electrolytes, electric charges are carried by charged atoms of the substance - ions.
- This process is called
- electrolysis.
- Faraday's Law:
- the mass of the substance released on the electrode during the time ∆t during the passage of an electric current is proportional to the current strength and time:
- m= kI∆t.
- This equation is called the law of electrolysis. The coefficient k, depending on the released substance, is called electrochemical equivalent of the substance.
- Since this chemical process takes a long time (in our experience – 30 minutes), copper (red deposit) is deposited on the cathode, released from the electrolyte. In this case, the electrolyte, instead of the copper molecules that went to the cathode, receives new copper molecules due to the dissolution of the second electrode - the anode.
- The phenomenon of electrolysis is applied in practice
- - for obtaining many metals from a salt solution;
- - for protection against oxidation or for decoration - various objects and machine parts are coated with thin layers of metals such as chrome, nickel, silver, gold;
- - in galvanoplasty – obtaining peelable coatings;
- - to obtain electronic boards (the basis of all electronic products);
- - to create copies from relief surfaces;
- - to obtain stereotypes for high-quality printed books.
- The experience of R. Tolman - T. Stew-art
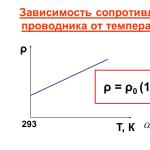
- Graph of specific resistance
- temperature dependent
- This is expressed by the formulas:
- R=R0(1+ αt) , ρ = ρ0 (1+αt).
- Here α is the temperature coefficient of resistance. Its values are very small and are defined in the resistivity table.
- For pure metals: α = 1/273 K-1.
- For alloys: 10-5 – 10-6 K -1
- Resistance thermometer
- This is the property of some materials to have strictly zero electrical resistance at
- they reach the temperature
- tours below a certain value. There are 26
- pure elements, alloys turning into superconductor
- current state.
- Gases in their normal state are dielectrics because they consist of electrically neutral atoms and molecules and therefore do not conduct electricity.
- Only ionized gases can be conductors,
- which contain electrons, positive and negative ions.
- In this case, the environment requires an external ionizer.
- The role of such an ionizer is played by heating and radiation.
- The passage of electric current through gases is called gas discharge.
- Non-self-sustaining gas discharge is a discharge that, having arisen in the presence of an electric field, can only exist under the influence of an external ionizer.
- Self discharge - a gas discharge in which current carriers arise as a result of processes in the gas that are caused by voltage applied to the gas.
- That is, this discharge continues even after the ionizer stops working.
- Varieties of this category:
- - spark;
- - arc;
- - crown;
- - smoldering.
- Spark discharge
- occurs between two electrodes charged with different charges and having a large potential difference. It is short-term, its mechanism is electronic shock.
- Lightning is a type of spark discharge.
- If, after receiving a spark discharge from a powerful source, the distance between the electrodes is gradually reduced, then the discharge from intermittent becomes continuous; a new form of gas discharge appears, called arc discharge .
- Lighting
- Welding
- Mercury arc.
- In highly inhomogeneous electric fields, formed, for example, between a tip and a plane or between a power line wire and the surface of the Earth, a special form of self-discharge occurs in gases,
- called corona discharge.
- Lightning rod(It is estimated that about 1,800 thunderstorms occur simultaneously in the atmosphere of the entire globe, producing an average of about 100 lightning strikes per second. Therefore, lightning protection is an important task).
- This is a discharge that occurs at low pressure.
- As the pressure decreases, the mean free path of the electron increases, and during the time between collisions it manages to acquire sufficient energy for ionization in an electric field with a lower intensity. The discharge is carried out by an electron-ion avalanche.
- Helium Neon Xenon
- 1. Application of electrolysis:
- https://fs00.infourok.ru/images/doc/161/185478/img7.jpg
- 2. Experience of T. Stewart - R. Tolman:
- https://fs00.infourok.ru/images/doc/86/103927/hello_html_m5ab75448.gif
- 3. Resistance graph:
- - https://ds04.infourok.ru/uploads/ex/0eea/000097a1-40f35dcb/310/img9.jpg
- 4. Electrometer:
- http://edufuture.biz/images/e/e5/A16.28.jpg
- 5. Zipper:
- http://thoughts-about-life.ru/wp-content/uploads/2012/02/molniya-1024x768.jpg
- 6.Arc discharge:
- http://sony.iiteco.ru/http/ftpfolder/Tesla/tesla1.jpg
- http://900igr.net/datai/fizika/Tok-v-razlichnykh-sredakh/0032-025-Dugovoj-razrjad.jpg
- 7.Corona discharge:
- https://www.estnauki.ru/images/stories/kor-razr.jpg
- http://turboz.ru/cmsdb/article_images/images/1194080299(1).jpg
- 8.Lightning rod:
- http://pandia.ru/text/77/296/images/image006_16.gif
- 9. Glow discharge:
- http://taurus-nsk.rf/wp-content/gallery/molnia_udarila_rightinbuttchicks/zashchita-ot-molnii-poselka.jpg
- 10. Physics: Textbook. for 10th grade general education institutions / G.Ya. Myakishev, B.B. Bukhovtsev, N.N. Sotsky. – 10th ed. – M.: Education, 2011. – 336 p.



Application of superconductors: Powerful electromagnets that operate without energy consumption. (Particle accelerators.) If it were possible to create superconducting materials at temperatures close to room temperature, lossless transmission of electricity would become possible.



Liquids: conductors (solutions of acids, alkalis and salts); conductors (solutions of acids, alkalis and salts); dielectrics (distilled water, kerosene...) dielectrics (distilled water, kerosene...) semiconductors (sulfide melts, molten selenium). semiconductors (sulfide melts, molten selenium).


The degree of dissociation (the proportion of molecules that have broken up into ions) Depends on: the concentration of the solution; solution concentration; dielectric constant of the solution; dielectric constant of the solution; temperature (increases with increasing temperature). temperature (increases with increasing temperature).

Electric current in liquids Directed movement of positive ions to the cathode and negative ions to the anode Directed movement of positive ions to the cathode and negative ions to the anode In liquid metals - movement of positive ions to the cathode and electrons to the anode. In liquid metals - the movement of positive ions to the cathode and electrons to the anode.




The mass of the substance released on the electrode when a charge of 1 C is transferred through the solution. The mass of the substance released on the electrode when a charge of 1 C is transferred through the solution. The ratio of the mass of an ion of a substance to its charge. The ratio of the mass of an ion of a substance to its charge.

Faraday's constant Faraday's constant The charge that must be passed through a solution of a 1-valent substance in order for 1 mole of a substance to be released at the electrode. A charge that must be passed through a solution of a 1-valent substance in order for 1 mole of the substance to be released at the electrode.


Application of electrolysis Electroplating (coating). Electroplating (coating). Galvanoplasty (making copies of relief objects). Galvanoplasty (making copies of relief objects). Refining (cleaning) of metals. Refining (cleaning) of metals. Obtaining pure metals from melts of natural compounds. Obtaining pure metals from melts of natural compounds.


Slide 2
Electric current can flow in five different media:
Metals Vacuum Semiconductors Liquids Gases
Slide 3
Electric current in metals:
Electric current in metals is the ordered movement of electrons under the influence of an electric field. Experiments show that when current flows through a metal conductor, no substance is transferred, therefore, metal ions do not take part in the transfer of electric charge.
Slide 4
The experiments of Tolman and Stewart provide evidence that metals have electronic conductivity
A coil with a large number of turns of thin wire was driven into rapid rotation around its axis. The ends of the coil were connected using flexible wires to a sensitive ballistic galvanometer G. The untwisted coil was sharply slowed down, and a short-term current arose in the circuit due to the inertia of the electrons.
Slide 5
Conclusion: 1.charge carriers in metals are electrons;
2. the process of formation of charge carriers - socialization of valence electrons; 3.current strength is directly proportional to voltage and inversely proportional to conductor resistance - Ohm’s law is satisfied; 4. technical application of electric current in metals: windings of motors, transformers, generators, wiring inside buildings, power transmission networks, power cables.
Slide 6
Electric current in a vacuum
Vacuum is a highly rarefied gas in which the mean free path of a particle is greater than the size of the vessel, that is, the molecule flies from one wall of the vessel to the other without colliding with other molecules. As a result, there are no free charge carriers in the vacuum, and no electric current occurs. To create charge carriers in a vacuum, the phenomenon of thermionic emission is used.
Slide 7
THERMAL ELECTRON EMISSION is the phenomenon of “evaporation” of electrons from the surface of a heated metal.
A metal spiral coated with metal oxide is brought into a vacuum, it is heated with an electric current (incandescent circuit) and electrons evaporate from the surface of the spiral, the movement of which can be controlled using an electric field.
Slide 8
The slide shows the inclusion of a two-electrode lamp
This lamp is called a vacuum diode
Slide 9
This electron tube is called a vacuum TRIOD.
It has a third electrode - a grid, the sign of the potential on which controls the flow of electrons.
Slide 10
Conclusions: 1. charge carriers – electrons;
2. the process of formation of charge carriers – thermionic emission; 3. Ohm's law is not fulfilled; 4.technical application – vacuum tubes (diode, triode), cathode ray tube.
Slide 11
Electric current in semiconductors
When heated or illuminated, some electrons become able to move freely within the crystal, so that when an electric field is applied, directional movement of electrons occurs. Semiconductors are a cross between conductors and insulators. Semiconductors are solid substances whose conductivity depends on external conditions (mainly heating and lighting).
Slide 12
As the temperature decreases, the resistance of metals decreases. In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators.
Dependence of resistivity ρ of a pure semiconductor on absolute temperature T.
Slide 13
Intrinsic conductivity of semiconductors
Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons. In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms. The valence electrons in a germanium crystal are much more strongly bound to the atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current.
Slide 14
Formation of an electron-hole pair
With increasing temperature or increasing illumination, some of the valence electrons may receive energy sufficient to break covalent bonds. Then free electrons (conduction electrons) will appear in the crystal. At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons. These vacancies are called “holes.”
Slide 15
Impurity conductivity of semiconductors
The conductivity of semiconductors in the presence of impurities is called impurity conductivity. There are two types of impurity conductivity - electronic and hole conductivity.
Slide 16
Electronic and hole conductivity.
If the impurity has a valence greater than the pure semiconductor, then free electrons appear. Conductivity – electronic, donor impurity, n-type semiconductor. If the impurity has a valence lower than that of the pure semiconductor, then bond breaks—holes—appear. Conductivity is hole, acceptor impurity, p-type semiconductor.
Slide 17
Conclusions: 1. charge carriers – electrons and holes;
2. the process of formation of charge carriers - heating, illumination or the introduction of impurities; 3.Ohm's law is not fulfilled; 4.technical application – electronics.
Slide 18
Electric current in liquids
Electrolytes are commonly called conducting media in which the flow of electric current is accompanied by the transfer of matter. The carriers of free charges in electrolytes are positively and negatively charged ions. Electrolytes are aqueous solutions of inorganic acids, salts and alkalis.
Slide 19
The resistance of electrolytes decreases with increasing temperature, since the number of ions increases with increasing temperature.
Graph of electrolyte resistance versus temperature.
Slide 20
Electrolysis phenomenon
This is the release on the electrodes of substances included in electrolytes; Positively charged ions (anions) under the influence of an electric field tend to the negative cathode, and negatively charged ions (cations) tend to the positive anode. At the anode, negative ions give up extra electrons (oxidation reaction) At the cathode positive ions receive the missing electrons (reductive).
Slide 21
Faraday's laws of electrolysis.
The laws of electrolysis determine the mass of a substance released during electrolysis at the cathode or anode during the entire period of passage of electric current through the electrolyte. k is the electrochemical equivalent of the substance, numerically equal to the mass of the substance released on the electrode when a charge of 1 C passes through the electrolyte.
Slide 22
Conclusion: 1. charge carriers – positive and negative ions;
2. process of formation of charge carriers – electrolytic dissociation; 3.electrolytes obey Ohm’s law; 4. Application of electrolysis: production of non-ferrous metals (removal of impurities - refining); electroplating - production of coatings on metal (nickel plating, chrome plating, gilding, silvering, etc.); electroplating - production of peelable coatings (relief copies).
Slide 23
Electric current in gases
Let's charge the capacitor and connect its plates to the electrometer. The charge on the capacitor plates remains indefinitely; there is no charge transfer from one capacitor plate to another. Therefore, the air between the capacitor plates does not conduct current. Under normal conditions, there is no conduction of electric current by any gases. Let us now heat the air in the gap between the plates of the condenser by introducing a lit burner into it. The electrometer will indicate the appearance of current, therefore, at high temperatures, part of the neutral gas molecules breaks up into positive and negative ions. This phenomenon is called gas ionization.
Slide 24
The passage of electric current through a gas is called a discharge.
The discharge that exists under the action of an external ionizer is not self-sustaining. If the action of the external ionizer continues, then after a certain time internal ionization (ionization by electron impact) is established in the gas and the discharge becomes independent.
Slide 25
Types of self-discharge:
SPARK GLOW CORONA ARC
Slide 26
Spark discharge
At a sufficiently high field strength (about 3 MV/m), an electric spark appears between the electrodes, which has the appearance of a brightly glowing winding channel connecting both electrodes. The gas near the spark heats up to a high temperature and suddenly expands, causing sound waves to appear and we hear a characteristic crackling sound.
Slide 27
Lightning. A beautiful and dangerous natural phenomenon - lightning - is a spark discharge in the atmosphere.
Already in the middle of the 18th century, it was suggested that thunderclouds carry large electrical charges and that lightning is a gigantic spark, no different except in size from the spark between the balls of an electric machine. This was pointed out, for example, by the Russian physicist and chemist Mikhail Vasilyevich Lomonosov (1711-1765), who, along with other scientific issues, dealt with atmospheric electricity.
Slide 28
Electric arc (arc discharge)
In 1802, Russian physicist V.V. Petrov (1761-1834) found that if you attach two pieces of charcoal to the poles of a large electric battery and, bringing the coals into contact, move them slightly apart, a bright flame will form between the ends of the coals, and the ends of the coals themselves will become white hot, emitting a blinding light.
Slide 30
Bibliography:
1. Kabardin O.F. Physics: Reference. materials. Textbook manual for students. – 5th ed., revised. and additional – M.: Education, 2003. website
View all slides
Slide 1
Presentation on the topic: “Electric current in various media” Performed by Alisa Kravtsova, ML No. 1, Magnitogorsk, 2009.Slide 2
 Electric current can flow in five different media: Metals Vacuum Semiconductors Liquids Gases
Electric current can flow in five different media: Metals Vacuum Semiconductors Liquids Gases
Slide 3
 Electric current in metals: Electric current in metals is the ordered movement of electrons under the influence of an electric field. Experiments show that when current flows through a metal conductor, no substance is transferred, therefore, metal ions do not take part in the transfer of electric charge.
Electric current in metals: Electric current in metals is the ordered movement of electrons under the influence of an electric field. Experiments show that when current flows through a metal conductor, no substance is transferred, therefore, metal ions do not take part in the transfer of electric charge.
Slide 4
 The experiments of Tolman and Stewart are proof that metals have electronic conductivity. A coil with a large number of turns of thin wire was driven into rapid rotation around its axis. The ends of the coil were connected using flexible wires to a sensitive ballistic galvanometer G. The untwisted coil was sharply slowed down, and a short-term current arose in the circuit due to the inertia of the electrons.
The experiments of Tolman and Stewart are proof that metals have electronic conductivity. A coil with a large number of turns of thin wire was driven into rapid rotation around its axis. The ends of the coil were connected using flexible wires to a sensitive ballistic galvanometer G. The untwisted coil was sharply slowed down, and a short-term current arose in the circuit due to the inertia of the electrons.
Slide 5
 Conclusion: 1.charge carriers in metals are electrons; 2. the process of formation of charge carriers - socialization of valence electrons; 3.current strength is directly proportional to voltage and inversely proportional to conductor resistance - Ohm’s law is satisfied; 4. technical application of electric current in metals: windings of motors, transformers, generators, wiring inside buildings, power transmission networks, power cables.
Conclusion: 1.charge carriers in metals are electrons; 2. the process of formation of charge carriers - socialization of valence electrons; 3.current strength is directly proportional to voltage and inversely proportional to conductor resistance - Ohm’s law is satisfied; 4. technical application of electric current in metals: windings of motors, transformers, generators, wiring inside buildings, power transmission networks, power cables.
Slide 6
 Electric current in a vacuum Vacuum is a highly rarefied gas in which the mean free path of a particle is greater than the size of the vessel, that is, the molecule flies from one wall of the vessel to the other without colliding with other molecules. As a result, there are no free charge carriers in the vacuum, and no electric current occurs. To create charge carriers in a vacuum, the phenomenon of thermionic emission is used.
Electric current in a vacuum Vacuum is a highly rarefied gas in which the mean free path of a particle is greater than the size of the vessel, that is, the molecule flies from one wall of the vessel to the other without colliding with other molecules. As a result, there are no free charge carriers in the vacuum, and no electric current occurs. To create charge carriers in a vacuum, the phenomenon of thermionic emission is used.
Slide 7
 THERMAL ELECTRON EMISSION is the phenomenon of “evaporation” of electrons from the surface of a heated metal. A metal spiral coated with metal oxide is brought into a vacuum, it is heated with an electric current (incandescent circuit) and electrons evaporate from the surface of the spiral, the movement of which can be controlled using an electric field.
THERMAL ELECTRON EMISSION is the phenomenon of “evaporation” of electrons from the surface of a heated metal. A metal spiral coated with metal oxide is brought into a vacuum, it is heated with an electric current (incandescent circuit) and electrons evaporate from the surface of the spiral, the movement of which can be controlled using an electric field.
Slide 8
 The slide shows the inclusion of a two-electrode lamp. This lamp is called a vacuum diode
The slide shows the inclusion of a two-electrode lamp. This lamp is called a vacuum diode
Slide 9
 This electron tube is called a vacuum TRIOD. It has a third electrode - a grid, the sign of the potential on which controls the flow of electrons.
This electron tube is called a vacuum TRIOD. It has a third electrode - a grid, the sign of the potential on which controls the flow of electrons.
Slide 10
 Conclusions: 1. charge carriers – electrons; 2. the process of formation of charge carriers – thermionic emission; 3.Ohm's law is not fulfilled; 4.technical application - vacuum tubes (diode, triode), cathode ray tube.
Conclusions: 1. charge carriers – electrons; 2. the process of formation of charge carriers – thermionic emission; 3.Ohm's law is not fulfilled; 4.technical application - vacuum tubes (diode, triode), cathode ray tube.
Slide 11
 Electric current in semiconductors When heated or illuminated, some electrons become able to move freely within the crystal, so that when an electric field is applied, directional movement of electrons occurs. Semiconductors are a cross between conductors and insulators. Semiconductors are solid substances whose conductivity depends on external conditions (mainly heating and lighting).
Electric current in semiconductors When heated or illuminated, some electrons become able to move freely within the crystal, so that when an electric field is applied, directional movement of electrons occurs. Semiconductors are a cross between conductors and insulators. Semiconductors are solid substances whose conductivity depends on external conditions (mainly heating and lighting).
Slide 12
 As the temperature decreases, the resistance of metals decreases. In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators. Dependence of resistivity ρ of a pure semiconductor on absolute temperature T.
As the temperature decreases, the resistance of metals decreases. In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators. Dependence of resistivity ρ of a pure semiconductor on absolute temperature T.
Slide 13
 Intrinsic conductivity of semiconductors Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons. In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms. The valence electrons in a germanium crystal are much more strongly bound to the atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current.
Intrinsic conductivity of semiconductors Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons. In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms. The valence electrons in a germanium crystal are much more strongly bound to the atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current.
Slide 14
 Formation of an electron-hole pair As the temperature increases or the illumination increases, some of the valence electrons may receive energy sufficient to break covalent bonds. Then free electrons (conduction electrons) will appear in the crystal. At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons. These vacancies are called “holes.”
Formation of an electron-hole pair As the temperature increases or the illumination increases, some of the valence electrons may receive energy sufficient to break covalent bonds. Then free electrons (conduction electrons) will appear in the crystal. At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons. These vacancies are called “holes.”
Slide 15
 Impurity conductivity of semiconductors The conductivity of semiconductors in the presence of impurities is called impurity conductivity. There are two types of impurity conductivity - electronic and hole conductivity.
Impurity conductivity of semiconductors The conductivity of semiconductors in the presence of impurities is called impurity conductivity. There are two types of impurity conductivity - electronic and hole conductivity.
Slide 16
 Electronic and hole conductivity. If the impurity has a valence greater than the pure semiconductor, then free electrons appear. Conductivity – electronic, donor impurity, n-type semiconductor. If the impurity has a valence lower than that of the pure semiconductor, then bond breaks—holes—appear. Conductivity is hole, acceptor impurity, p-type semiconductor.
Electronic and hole conductivity. If the impurity has a valence greater than the pure semiconductor, then free electrons appear. Conductivity – electronic, donor impurity, n-type semiconductor. If the impurity has a valence lower than that of the pure semiconductor, then bond breaks—holes—appear. Conductivity is hole, acceptor impurity, p-type semiconductor.
Slide 17
 Conclusions: 1. charge carriers – electrons and holes; 2. the process of formation of charge carriers - heating, illumination or the introduction of impurities; 3.Ohm's law is not fulfilled; 4.technical application – electronics.
Conclusions: 1. charge carriers – electrons and holes; 2. the process of formation of charge carriers - heating, illumination or the introduction of impurities; 3.Ohm's law is not fulfilled; 4.technical application – electronics.
Slide 18
 Electric current in liquids Electrolytes are commonly called conducting media in which the flow of electric current is accompanied by the transfer of matter. The carriers of free charges in electrolytes are positively and negatively charged ions. Electrolytes are aqueous solutions of inorganic acids, salts and alkalis.
Electric current in liquids Electrolytes are commonly called conducting media in which the flow of electric current is accompanied by the transfer of matter. The carriers of free charges in electrolytes are positively and negatively charged ions. Electrolytes are aqueous solutions of inorganic acids, salts and alkalis.
Slide 19
 The resistance of electrolytes decreases with increasing temperature, since the number of ions increases with increasing temperature. Graph of electrolyte resistance versus temperature.
The resistance of electrolytes decreases with increasing temperature, since the number of ions increases with increasing temperature. Graph of electrolyte resistance versus temperature.
Slide 20
 The phenomenon of electrolysis is the release of substances included in electrolytes on the electrodes; Positively charged ions (anions) under the influence of an electric field tend to the negative cathode, and negatively charged ions (cations) tend to the positive anode. At the anode, negative ions give up extra electrons (oxidation reaction). At the cathode, positive ions receive the missing electrons (reduction reaction).
The phenomenon of electrolysis is the release of substances included in electrolytes on the electrodes; Positively charged ions (anions) under the influence of an electric field tend to the negative cathode, and negatively charged ions (cations) tend to the positive anode. At the anode, negative ions give up extra electrons (oxidation reaction). At the cathode, positive ions receive the missing electrons (reduction reaction).
Slide 21
 Faraday's laws of electrolysis. The laws of electrolysis determine the mass of a substance released during electrolysis at the cathode or anode during the entire period of passage of electric current through the electrolyte. k is the electrochemical equivalent of the substance, numerically equal to the mass of the substance released on the electrode when a charge of 1 C passes through the electrolyte.
Faraday's laws of electrolysis. The laws of electrolysis determine the mass of a substance released during electrolysis at the cathode or anode during the entire period of passage of electric current through the electrolyte. k is the electrochemical equivalent of the substance, numerically equal to the mass of the substance released on the electrode when a charge of 1 C passes through the electrolyte.
Slide 22
 Conclusion: 1. charge carriers – positive and negative ions; 2. the process of formation of charge carriers - electrolytic dissociation; 3.electrolytes obey Ohm’s law; 4. Application of electrolysis: production of non-ferrous metals (removal of impurities - refining); electroplating - obtaining coatings on metal (nickel plating, chrome plating, gold plating, silver plating, etc.); galvanoplasty - producing peelable coatings (relief copies).
Conclusion: 1. charge carriers – positive and negative ions; 2. the process of formation of charge carriers - electrolytic dissociation; 3.electrolytes obey Ohm’s law; 4. Application of electrolysis: production of non-ferrous metals (removal of impurities - refining); electroplating - obtaining coatings on metal (nickel plating, chrome plating, gold plating, silver plating, etc.); galvanoplasty - producing peelable coatings (relief copies).
Slide 23
 Electric current in gases Let's charge the capacitor and connect its plates to the electrometer. The charge on the capacitor plates remains indefinitely; there is no charge transfer from one capacitor plate to another. Therefore, the air between the capacitor plates does not conduct current. Under normal conditions, there is no conduction of electric current by any gases. Let us now heat the air in the gap between the plates of the condenser by introducing a lit burner into it. The electrometer will indicate the appearance of current, therefore, at high temperatures, part of the neutral gas molecules breaks up into positive and negative ions. This phenomenon is called gas ionization.
Electric current in gases Let's charge the capacitor and connect its plates to the electrometer. The charge on the capacitor plates remains indefinitely; there is no charge transfer from one capacitor plate to another. Therefore, the air between the capacitor plates does not conduct current. Under normal conditions, there is no conduction of electric current by any gases. Let us now heat the air in the gap between the plates of the condenser by introducing a lit burner into it. The electrometer will indicate the appearance of current, therefore, at high temperatures, part of the neutral gas molecules breaks up into positive and negative ions. This phenomenon is called gas ionization.
Slide 1
Presentation on the topic: “Electric current in various media”
Performed by Alisa Kravtsova, ML No. 1, Magnitogorsk, 2009.

Slide 2
Electric current can flow in five different media:
Metals Vacuum Semiconductors Liquids Gases

Slide 3
Electric current in metals:
Electric current in metals is the ordered movement of electrons under the influence of an electric field. Experiments show that when current flows through a metal conductor, no substance is transferred, therefore, metal ions do not take part in the transfer of electric charge.

Slide 4
The experiments of Tolman and Stewart provide evidence that metals have electronic conductivity
A coil with a large number of turns of thin wire was driven into rapid rotation around its axis. The ends of the coil were connected using flexible wires to a sensitive ballistic galvanometer G. The untwisted coil was sharply slowed down, and a short-term current arose in the circuit due to the inertia of the electrons.

Slide 5
Conclusion: 1.charge carriers in metals are electrons;
2. the process of formation of charge carriers - socialization of valence electrons; 3.current strength is directly proportional to voltage and inversely proportional to conductor resistance - Ohm’s law is satisfied; 4. technical application of electric current in metals: windings of motors, transformers, generators, wiring inside buildings, power transmission networks, power cables.

Slide 6
Electric current in a vacuum
Vacuum is a highly rarefied gas in which the mean free path of a particle is greater than the size of the vessel, that is, the molecule flies from one wall of the vessel to the other without colliding with other molecules. As a result, there are no free charge carriers in the vacuum, and no electric current occurs. To create charge carriers in a vacuum, the phenomenon of thermionic emission is used.

Slide 7
THERMAL ELECTRON EMISSION is the phenomenon of “evaporation” of electrons from the surface of a heated metal.
A metal spiral coated with metal oxide is brought into a vacuum, it is heated with an electric current (incandescent circuit) and electrons evaporate from the surface of the spiral, the movement of which can be controlled using an electric field.

Slide 8
The slide shows the inclusion of a two-electrode lamp
This lamp is called a vacuum diode

Slide 9
This electron tube is called a vacuum TRIOD.
It has a third electrode - a grid, the sign of the potential on which controls the flow of electrons.

Slide 10
Conclusions: 1. charge carriers – electrons;
2. the process of formation of charge carriers – thermionic emission; 3.Ohm's law is not fulfilled; 4.technical application - vacuum tubes (diode, triode), cathode ray tube.

Slide 11
Electric current in semiconductors
When heated or illuminated, some electrons become able to move freely within the crystal, so that when an electric field is applied, directional movement of electrons occurs. Semiconductors are a cross between conductors and insulators.
Semiconductors are solid substances whose conductivity depends on external conditions (mainly heating and lighting).

Slide 12
As the temperature decreases, the resistance of metals decreases. In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators.
Dependence of resistivity ρ of a pure semiconductor on absolute temperature T.

Slide 13
Intrinsic conductivity of semiconductors
Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons. In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms. The valence electrons in a germanium crystal are much more strongly bound to the atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current.

Slide 14
Formation of an electron-hole pair
With increasing temperature or increasing illumination, some of the valence electrons may receive energy sufficient to break covalent bonds. Then free electrons (conduction electrons) will appear in the crystal. At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons. These vacancies are called “holes.”

Slide 15
Impurity conductivity of semiconductors
The conductivity of semiconductors in the presence of impurities is called impurity conductivity. There are two types of impurity conductivity - electronic and hole conductivity.

Slide 16
Electronic and hole conductivity.
If the impurity has a valence greater than the pure semiconductor, then free electrons appear. Conductivity – electronic, donor impurity, n-type semiconductor.
If the impurity has a valence lower than that of the pure semiconductor, then bond breaks—holes—appear. Conductivity is hole, acceptor impurity, p-type semiconductor.

Slide 17
Conclusions: 1. charge carriers – electrons and holes;
2. the process of formation of charge carriers - heating, illumination or the introduction of impurities; 3.Ohm's law is not fulfilled; 4.technical application – electronics.

Slide 18
Electric current in liquids
Electrolytes are commonly called conducting media in which the flow of electric current is accompanied by the transfer of matter. The carriers of free charges in electrolytes are positively and negatively charged ions. Electrolytes are aqueous solutions of inorganic acids, salts and alkalis.

Slide 19
The resistance of electrolytes decreases with increasing temperature, since the number of ions increases with increasing temperature.
Graph of electrolyte resistance versus temperature.

Slide 20
Electrolysis phenomenon
This is the release on the electrodes of substances included in electrolytes; Positively charged ions (anions) under the influence of an electric field tend to the negative cathode, and negatively charged ions (cations) tend to the positive anode. At the anode, negative ions give up extra electrons (oxidation reaction). At the cathode, positive ions receive the missing electrons (reduction reaction).

Slide 21
Faraday's laws of electrolysis.
The laws of electrolysis determine the mass of a substance released during electrolysis at the cathode or anode during the entire period of passage of electric current through the electrolyte.
k is the electrochemical equivalent of the substance, numerically equal to the mass of the substance released on the electrode when a charge of 1 C passes through the electrolyte.

Slide 22
Conclusion: 1. charge carriers – positive and negative ions;
2. the process of formation of charge carriers - electrolytic dissociation; 3.electrolytes obey Ohm’s law; 4. Application of electrolysis: production of non-ferrous metals (removal of impurities - refining); electroplating - obtaining coatings on metal (nickel plating, chrome plating, gold plating, silver plating, etc.); galvanoplasty - producing peelable coatings (relief copies).

Slide 23
Electric current in gases
Let's charge the capacitor and connect its plates to the electrometer. The charge on the capacitor plates remains indefinitely; there is no charge transfer from one capacitor plate to another. Therefore, the air between the capacitor plates does not conduct current. Under normal conditions, there is no conduction of electric current by any gases. Let us now heat the air in the gap between the plates of the condenser by introducing a lit burner into it. The electrometer will indicate the appearance of current, therefore, at high temperatures, part of the neutral gas molecules breaks up into positive and negative ions. This phenomenon is called gas ionization.